COVID-19 TESTING
Testing for &&4& becomes a very important public health measure during this &&5& time.
There are many different tests for &&4& patients worldwide. &&11& takes you into details of these tests.
Global Wellness Force, April 2020
The authoritative identification or gold standard method for detection of &&12& that causes &&4& is viral culture from respiratory
aspirate or blood and antigen or molecular test with high-throughput whole genome sequencing method from nasal or oral swab. Unfortunately,
these tests are not very practical in clinical settings especially in dealing with current &&5& state. The viral culture takes very long time for the result and
the High-throughput whole genome sequencing depends heavily on high cost equipment. From clinical practicality standpoint, there are 2 categories for &&4& testing:
1. Molecular or antigen test: real-time quantitative or qualitative polymerase chain reaction ( PCR ) to detect the &&12& particles, and
2. Serology or antibody test to detect human antibodies, immunoglobulin (Ig) M and G qualitatively or quantitatively to detect body response to &&4&.
To this date, there are 40 real-time qualitative PCR molecular or antigen tests that have been approved by FDA under its emergency use authorization (EUA) and 4
qualitative IgM and IgG serology or antibody tests to detect antibodies against the &&12&.
In term of test results, for example the QuestTM and LabCorpTM PCR tests turned around time around 3-4 days, and the newer ID Now &&4& test developed by Abbott LaboratoryTM has a turned around time of less than 15 minutes.
The CellexTM IgG/IgM test turned around time is less than 45 minutes. There are many more of similar molecular and serology tests out in the world
that are currently not authorized by the US Food and Drug Administration (FDA).
The molecular real-time qualitative PCR tests are using nasal or oral swab specimens and the serology or antibody tests are using capillary fingerstick whole blood specimens.
A list of these tests on FDA website:
https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations#covid19ivd
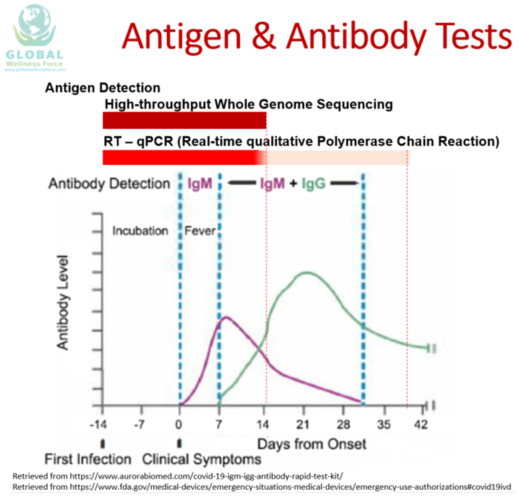
The graphic here will help you understand the capability of the antigen and antibody tests to detect the presence of &&4& based on duration of the illness.
Please note here that we are making the assumption that all these tests have great sensitivity and specificity.
The very top part of the graphic shows the antigen tests, for a reference,
we include the high-throughput whole genome sequencing test, which is impractical in &&5& state, in addition to the more practical real-time qualitative PCR test.
The Y axis indicates the body serum antibody or Ig M and IgG levels. The X axis indicates the timing of illness.
Please note here that the point 0 is where patient starts to have first symptom which typically is fever.
This graphic shows that the Antigen test, either the high-throughput whole genome sequencing or real time qualitative PCR tests,
will be positive from the day the patient is exposed to &&4& which can be as early as 14 days prior to onset of symptoms which is so called the disease incubation period.
The high-throughput whole genome sequencing test will remain positive until the virus is completely eradicated from the body while the real-time qualitative PCR may
remain positive for a longer period of time after the patients has recovered from the illness as these patients may still shed the remnants of virus particle which are no longer infectious or contagious.
The human body will typically produce its first antibody against the virus, Ig M, at the onset of symptoms and peak at approximately day 7 after the onset of symptom.
A longer lasting antibody, IgG, will form at approximately day 7 after the onset of fever and peak at approximately day 21 following the onset of symptom.
Ig G will remain detected in the body for longer period which typically about 6 months or longer.
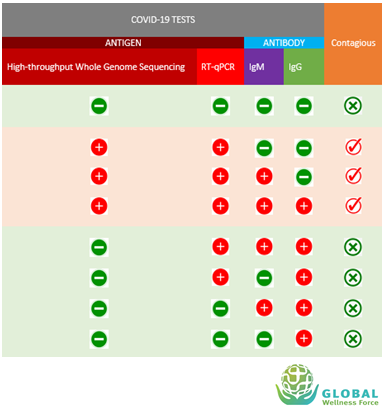
This table here sums up the prior graphic and shows all possible combinations of molecular and serology test results and the possibility of &&4& patients being contagious to others.
The table may help healthcare providers or authorities to decide when a person is no longer infectious or contagious and when
it is safe to lift the quarantine, isolation or social distancing order.
If all the antigen and antibody tests are negative, it means the person has never contracted &&4& and not contagious but may potentially get infected if
exposed to the virus as that person has no immunity towards &&4&. Negative antigen detection using PCR after recovering from &&4& means that
the person is no longer contagious, however positive PCR result does not necessarily mean that the person remains contagious as the PCR may
still detect remnants of virus particle that remains in patient’s body after recovering from the illness but this particle would not infect others.
A person with antibody IgG positive and a negative PCR tells us that this person has developed long term immunity against the &&4& and no longer contagious.
For a reference only, positive result from high-throughput whole genome sequencing test indicates the patient may still shed the virus and negative result indicates
patients is no longer shedding the virus. Unfortunately, this high-throughput whole genome sequencing test in clinically not practical.
There are many other tests that are considered non-definitive testing that may help physicians to diagnose &&4& patient, to predict disease severity
as well as to follow the progression of the illness. These tests are mostly done for &&4& patients admitted to the hospital. Following are several abnormal
blood tests that have been reported by clinicians from hospital worldwide taking care of &&4& patients: leukopenia (decreased white blood cell count),
lymphopenia (low lymphocyte count), thrombocytopenia (low platelet count), elevated NLR (neutrophil to lymphocyte ratio).
All these three later tests have been linked to more severe cases. Patients may also have elevated serum lactate dehydrogenase (LDH),
elevated liver function test (increasing liver specific serum alanine aminotransferase or ALT and less liver specific serum aspartate aminotransferase or AST) levels.
Patients with more severe cases also showed elevations of their serum inflammatory markers such as C-reactive protein (CRP) of more than ten times acceptable normal value,
serum ferritin of more than 1000 ng/ml, serum InterLeukin-6 (IL-6) of more than 40 to 100 pg/ml and serum procalcitonin. Other abnormal blood tests like increasing serum d-dimer
and lymphopenia have been associated with higher mortality.
Besides blood Tests, radiology or imaging studies have also been frequently used in management of &&4& patients, such as chest x-ray and chest computed tomography (CT) scan.
Chest x-ray of patient with &&4& shows bilateral air-space consolidation. Chest CT-scan of patient with &&4& shows bilateral multi lobar ground-glass opacifications with a peripheral,
asymmetric and posterior distribution.
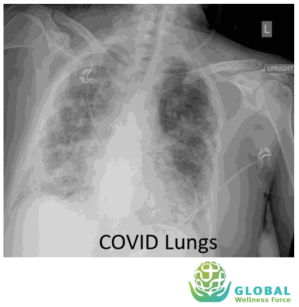
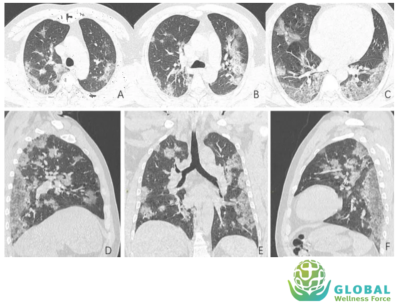
While imaging studies are important in helping clinicians caring for &&4& patients, given the great variability of these findings, chest imaging studies alone is not recommended for the diagnosis of &&4&.
